Molecule Shape Of Bcl3
Signs and symptoms of acute ingestion of boron trichloride may be severe and include salivation intense thirst difficulty in swallowing chills pain and shock. The molecular structure of BCl 3 features Boron as the central atom and three Chlorine atoms surrounding it.
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape
What is the molecular geometry of BCl3.

Molecule shape of bcl3. Back to Molecular Geometries Polarity Tutorial. Signs and Symptoms of Acute Boron Trichloride Exposure. This video tutorial will explain how to draw the Lewis dot structure and molecular geometry for boron trichloride BCl3.
The bond angle is. A Lewis structure is first structure and has two extra lone pairs on the central atom. Use VSEPR table to find the shape.
4To minimise repulsions the electrons tend to occupy positions with. The molecular geometry of a molecule describes the three-dimensional shape of just the atoms. What is the value of the bond angles in BCl3.
Therefore there exists no polarization of charges across the BCl3 molecule. AX 3 has trigonal planar shape. BCl3 is SP2 hybridisation and hence its structure is trigonal planner.
Molecular Geometry Polarity Tutorial. The molecular geometry of the BCl3 molecule is __________ and this molecule is __________. For the above molecule VSEPR notation will be AX 3 E 0.
Youll find the correct answer below. How do I determine the bond angle in a molecule. The molecular geometry of the BCl3 molecule is _____ and this molecule is _____.
Download a copy of VSEPR shapes table here Bond angle in BCl 3 Bond angle of Cl-B-Cl covalent bond in BCl 3 molecule is 120The representation is shown below. Therefore this molecule is nonpolar. Here is the answer for the question The molecular geometry of the BCl3 molecule is __________ and this molecule is __________.
Chemistry questions and answers. Which of the following has a T-shaped structure. For more information you must also go through an article on the polarity of BCl3.
Which of the following has square-planar molecular geometry. BCl3 molecule has trigonal planar structure with bond angle 120. So the shape of BCl 3 molecule is trigonal planar.
C Molecular shape is T shaped. The molecular geometry of BCl3 is trigonal pyramidal. Due to the presence of lone pairs the Chlorine atoms repel each other forming bond angles of 120.
Boron Trichloride on Wikipedia. This is because the molecular arrangement of the chlorine atom is part of a complete triangular shape. The bond angle is 120o.
Draw its VSEPR and Lewis structure. Oral esophageal and stomach burns are common. 1 The Molecular Geometry Of The BCl3 Molecule Is And This Molecule Is - A Trigonal Pyramidal Polar B Trigonal Pyramidal Nonpolar C Trigonal Planar Polar D Trigonal Planar Nonpolar E Trigonal Bipyramidal Polar 2 Which One Of.
Which series correctly identifies the hybridization of the central atom in a molecule. Chemistry Molecular Orbital Theory Molecular Geometry. The shape of the molecule depends on the number of bonded and non bonded electron pairs around central atom.
Jul 12 2014 Answer link. B VSEPR 3 bp 2 lp 5 shape is trigonal bipyramidal. This is free chemistry help for you.
The boron is located in the center which has three valence electrons and balances out the three chlorine. 1 Answer Humaam H. The Correct Answer is 120 degreesFor the molecules in which there are no lone pairs of electrons on the central atom the electronic geometry is the same as the molecular geometry.
1 The Molecular Geometry Of The BCl3 Molecule Is And This Molecule Is - A Trigonal Pyramidal. The BCl3 molecule is considered to be non-polar because the charge distribution across the molecule is uniform as the shape of the molecule is symmetric ie. 3There is repulsion between electrons in valence shell as they are negatively charged.
A trigonal pyramidal polar B trigonal pyramidal nonpolar C trigonal planar polar D trigonal planar nonpolar E trigonal bipyramidal polar. If we look at the structure BC l3. 5 rows If we look at the structure BCl 3 molecular geometry is trigonal planar.
The molecular geometry of BCl 3 is trigonal planar with symmetric charge distribution around the central atom. Molecular geometry is trigonal planar.
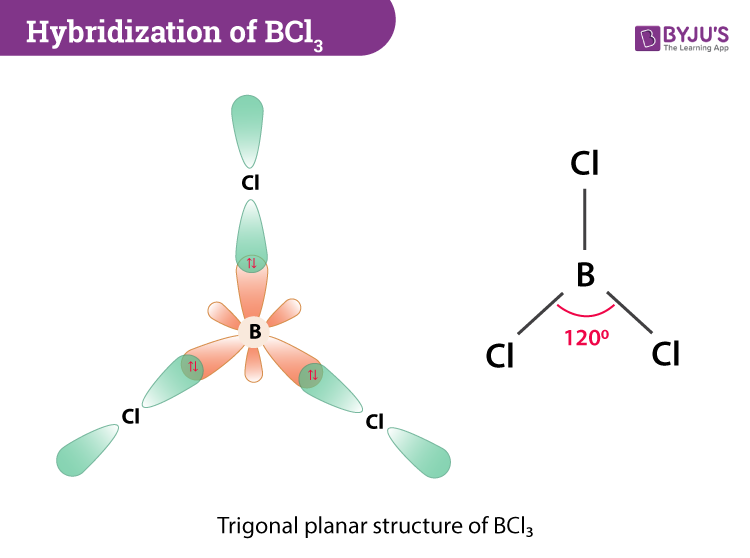
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have
What Is The Structure Of Bcl3 Quora

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
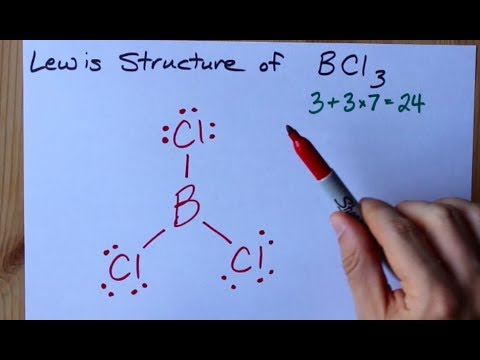
How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Bcl3 Lewis Structure How To Draw The Lewis Structure For Bcl3 Youtube
0 Response to "Molecule Shape Of Bcl3"
Post a Comment